Dr Meng Wang and members of the Welch Group have been working with colleagues in the Department of Chemistry, the University of Exeter and the University of Eastern Finland to study the biochemistry of Pseudomonas aeruginosa. This bacteria causes hard-to-treat lung infections in people with Cystic Fibrosis and it can become antibiotic-resistant and lead to long-term infection if not treated early. The resistant strains are classified Priority 1 Pathogens and in urgent need of new treatments by the World Health Organisation.
A new paper ‘Pseudomonas aeruginosa acyl-CoA dehydrogenases and structure-guided inversion of their substrate specificity’, published in Nature Communications identifies the key enzymes involved in breaking down fatty acids in P. aeruginosa. Professor Martin Welch said:
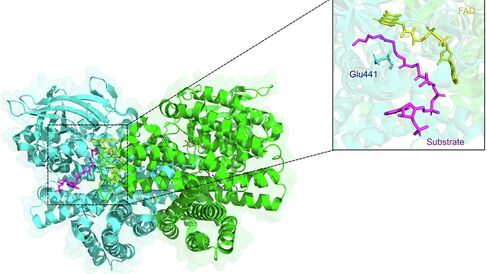
‘One of the main reasons why P. aeruginosa colonises the CF airways is because they are packed full of fatty acids, and P. aeruginosaloves fatty acids. However, the key enzymes involved in breaking these compounds down have eluded us for many years. In this work, and with the generous support of the CF Trust, we used state-of-the-art approaches to identify these enzymes, and show that indeed, they play an important role in infection. We also show that the enzymes can be inhibited by small molecules, opening the way towards targeting the pathway with drugs.’
This important research identifies the two enzymes that are used by P. aeruginosa and mobilises the latest structural and computational analysis techniques to find chemicals that block the activity of these enzymes. This is a crucial step in developing new medicines to treat Pseudomonas aeruginosa infections.
The work is funded by the Cystic Fibrosis Trust, and Dr Lucy Allen, Director of Research and Healthcare Data, said:
‘We know that many people with CF live with long term lung infections caused by Pseudomonas aeruginosa and we desperately need better, more effective ways to treat them. We’re delighted that our investment in this area of research has generated such important results, and brought our goal of developing more effective treatments for CF infections one step closer.’
