The Hyvönen Group, and collaborators at the University of Cincinnati, have published a new paper in The EMBO Journal.
Cell signalling in the broadest terms is any communication process that governs and coordinates the activities and actions of a cell, including the process by which cells sense and respond to their neighbours and environment. It is this capability of cells perceiving and adapting to their surroundings through signalling that forms the basis of cellular development, tissue repair, immunity and many other essential cellular processes. As such, errors in cell signalling pathways are responsible for numerous diseases; notably cancer, autoimmunity and diabetes. These pathological outcomes of abnormal cell signalling highlight the fundamental importance of signalling regulation for normal biology.
A common method for regulating cell signalling involves cells initially producing signalling proteins in an inactive, or latent form. Activation of the signalling proteins then only occurs where and when a signalling response is required, often through removal (proteolytic cleavage) of an inactivating 'off-switch' region (pro-domain) of the protein via an enzyme known as a protease.
Myostatin, a member of the transforming growth factor β (TGF-β) superfamily of signalling proteins and a suppressor of muscle growth, is regulated through this process of proteolytic cleavage. It is first produced inside cells as an inactive precursor (pro-myostatin). Pro-myostatin pairs are then secreted outside of the cell to the extracellular matrix and the pro-domains cleaved off. In this case, however, the cleaved pro-domains remain associated with myostatin, holding it in an inactive state through preventing its binding to receptors. For activation, a second proteolytic cleavage event occurs within the pro-domains, subsequently allowing the mature myostatin to initiate signalling.1-6
As an inhibitor of skeletal muscle growth, there is significant interest in determining the complete molecular mechanism of myostatin activation for public health benefits. Manipulation of myostatin signalling could, for example, be used to increase muscle mass in patients of muscular atrophic disorders such as muscular dystrophy and cancer-associated muscle loss.1,7-8 However, targeted inhibition of myostatin signalling to promote muscle growth has proved difficult, with a number of potential treatments failing to meet primary clinical endpoints in phase II trials, and no myostatin inhibitors currently approved for clinical use.8-10 This is likely due to these inhibitors exerting an unwanted effect on the functions of other TGF-β family members with similar protein structures to mature myostatin, and the narrow time and spatial window during which mature myostatin functions.
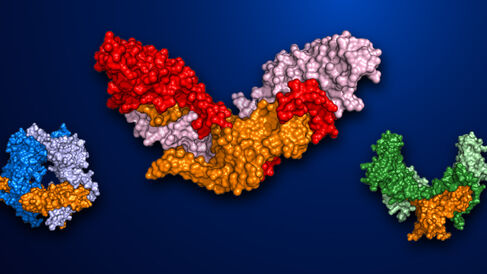
In their new paper, Cotton et al. utilise X-ray crystallography to determine the structure of pro-myostatin, finding that it adopts an unexpected 'open-armed' conformation, distinct from the structure of inactive pro-TGF-β. Subsequent analysis of this structure, and accompanying mutagenesis experiments, have allowed identification of a number of key features necessary for myostatin inactivation, and for the process of its controlled activation. In addition to enhancing our understanding of myostatin regulation, the discovery that pro-myostatin has a radically different structure to that of pro-TGF-β provides a basis for development of the next generation of myostatin inhibitors that target the latent form of the protein, and/or its proteolytic cleavage, instead of the mature form.
1McPherron et al., Proc. Natl. Acad. Sci. U.S.A.94:12457 (1997).
2Lee and McPherron, Proc. Natl. Acad. Sci. U.S.A.98:9306 (2001).
3Jiang et al., Biochem. Biophys. Res. Commun. 315:525 (2004).
4Wolfman et al., Proc. Natl. Acad. Sci. U.S.A.100:15842 (2003).
5Sengle et al., J. Biol. Chem. 283:13874 (2008).
6Shi et al., Nature474:343 (2011).
7McPherron et al., Nature387:83 (1997).
8Smith and Lin, Curr. Opin. Support. Palliat. Care7:352 (2013).
9Novartis, 2016 Q1 Financial Report (2016).
10Atara Bio, Atara Bio Conference Call in (2015).
