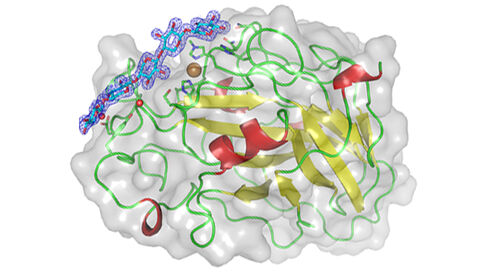
The Dupree Group have published a paper in Nature Communications offering insight into enzymes that break down plant cell walls.
With the world's population predicted to reach 8 billion by 2024 and our gradual depletion of non-renewable energy resources, the need for sustainable sources of energy and material is ever increasing. Our most abundant source of renewable energy and biomaterial is lignocellulose (dry plant matter), which is composed of cellulose, hemicellulose and lignin.1-2 This mixed composition of carbohydrates, however, makes lignocellulose difficult to work with and refine for use.3-4
Several potential methods have been proposed to overcome the difficulties in utilising lignocellulose, including chemical, mechanical and enzymatic approaches. Recent advances in enzyme mix formulas have found that LPMOs (lytic polysaccharide monooxygenases) accelerate breakdown of lignocellulose within the plant cell wall, and are thus assisting cellulosic-ethanol bio-refineries move towards being commercially and environmentally viable.5-6
LPMOs are a family of enzymes found within fungi and some bacteria that oxidatively break down carbohydrate chains into individual sugar units. However, the mechanism by which LPMO enzymes break down different carbohydrate chains is poorly understood.
In their new paper, a collaboration of several European labs in the CESBIC (Critical Enzymes for Sustainable Biofuels from Cellulose) consortium, including the Dupree Group, investigate two AA9-type LPMO enzymes, using X-ray crystallography to determine their structures when bound with different carbohydrate chains. Their results show how interactions of the carbohydrate with the enzyme active site determine where the carbohydrate chain will be broken. Specifically, they report LsAA9A and CvAA9A to both cleave a range of cellulosic and non-cellulosic substrates, but with different substrate specificity and site of attack on the carbohydrate, depending on protein-carbohydrate interactions at the binding surface, and on the electronic activation of copper in the enzyme active site. Combinations of mechanisms of function across LPMO enzymes likely greatly extend their range of potential substrates, and can offer novel insights into their biochemical mode of action for bioenergy and biomaterial research.
1Carroll and Somerville, Annu. Rev. Plant. Biol. 60:165, 2009.
2Perlack and Stokes, Technical Report ORNL/TM-2011/224, 227 (US Department of Energy), 2011.
3Marriott et al., New. Phytol. 209:1366, 2016.
4Simmons et al., Nat. Commun. 7:13902, 2016.
5Correa et al., Appl. Microbiol. Biotechnol. 100:9, 2016.
6Muller et al., Biotechnol. Biofuels. 8:817, 2015.
